
Proper synthesis, folding, subcellular targeting, and catabolism of proteins and peptides, therefore, are essential for human health.
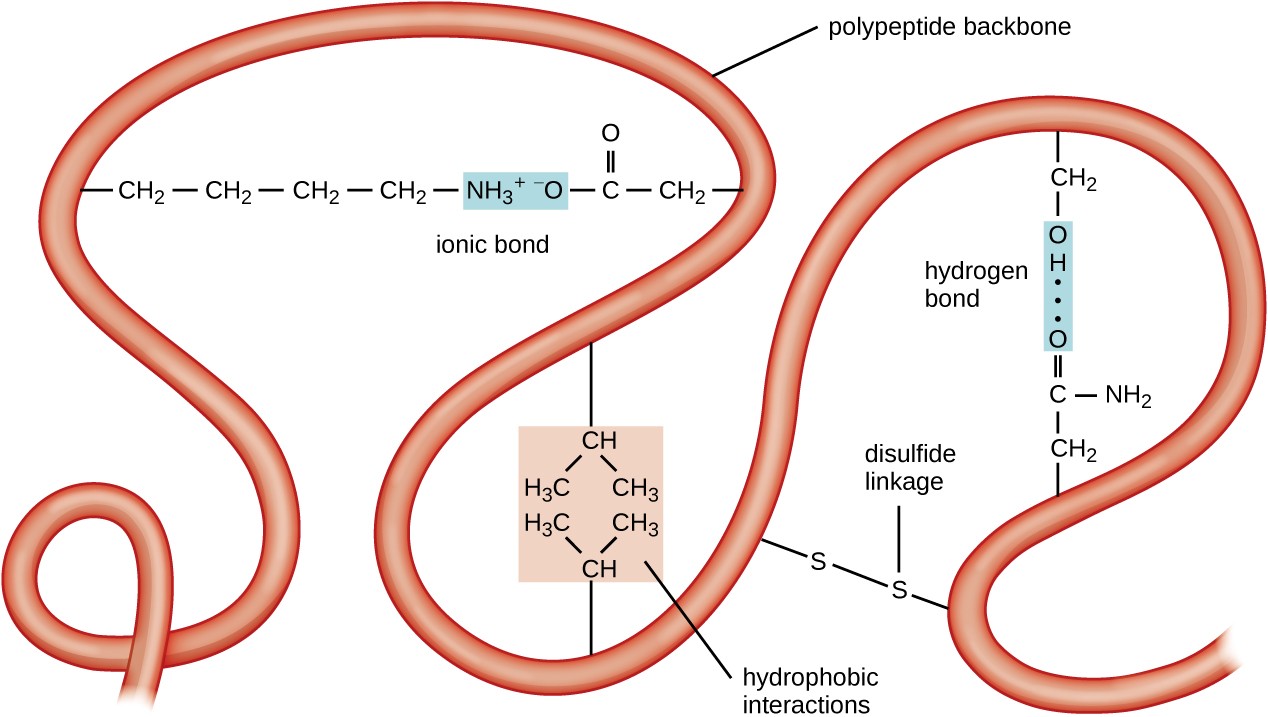
Systems of short peptides, larger protein monomers, and multimeric protein complexes are the tools that orchestrate and control human physiologic and biochemical processes. Proteome diversity arises from linear amino acid sequence and an array of modifications that include acylation, phosphorylation, glycosylation, and isoprenylation. The human genome contains the information to dictate formation of approximately 20,000 polypeptides, but the actual diversity of the human proteome and peptidome is manifold more expansive. Polymers of amino acids may be relatively short (peptides) or long (proteins). This chapter first describes the chemistry, metabolism, transport, and analysis of amino acids. The catalog of amino acids, peptides, and proteins in various biological fluids is a target-rich environment for the detection of pathological states. The polymers of amino acids, peptides, and proteins orchestrate and control the vast array of human physiologic and biochemical processes. Amino acids are classified as hydrophilic when they seek contact with aqueous solutions.Amino acids are the building blocks of proteins, but they also play diverse roles in the provision of energy and the formation of a number of other important biomolecules, including hormones, neurotransmitters, and signaling molecules. Hydrophilic amino acids are organic molecules that form proteins when linked together with other amino acids. Positively charged (basic amino acids non-acidic amino acids) Polar Hydrophilic pK=10.5īeside this, which amino acid is most polar?Īrginine and Lysine are pretty much tied for most polar.
Subsequently, question is, is lysine hydrophobic or hydrophilic? Amino acid poperties Amino-acid name Shown at the right is the structure of valine. Hydrophobic Amino Acids The nine amino acids that have hydrophobic side chains are glycine ( Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine ( Ile), proline (Pro), phenylalanine (Phe), methionine (Met), and tryptophan (Trp). Likewise, people ask, which amino acids are hydrophobic? Amino acids are ordered from the most hydrophobic one, Isoleucine (I, on the left hand side) to the most hydrophilic one, Arginine (R, on the right hand side), according to the Kyte-Doolitle scale.


 0 kommentar(er)
0 kommentar(er)
